Symptom Monitoring: SMaRRT-HD
Improving Hemodialysis-Related Symptoms
What challenge are we trying to solve?
Hemodialysis is a life-saving treatment but often causes distressing symptoms. People treated with hemodialysis frequently report symptoms such as muscle cramps, itching, and fatigue, and these symptoms contribute to poor quality of life and hospitalizations. Research in patients with other chronic diseases show that frequently asking patients to report their symptoms using standardized questionnaires (patient-reported outcome measures) and helping medical providers follow up on reported symptoms can improve symptoms, quality of life, and health care use. In current hemodialysis practice in the United States, patients formally report their symptoms once a year on a quality of life survey, and there is no system to help medical providers follow up on the reported symptoms.
“When I got home from dialysis, I would have to sleep for a few hours before I could function. But that whole day was really done. After that, I could go about my chores or do what I had to do – but the next day, I would have to go through it all over again. So, it really sucks up your whole life.”
SMaRRT-HD Study Overview
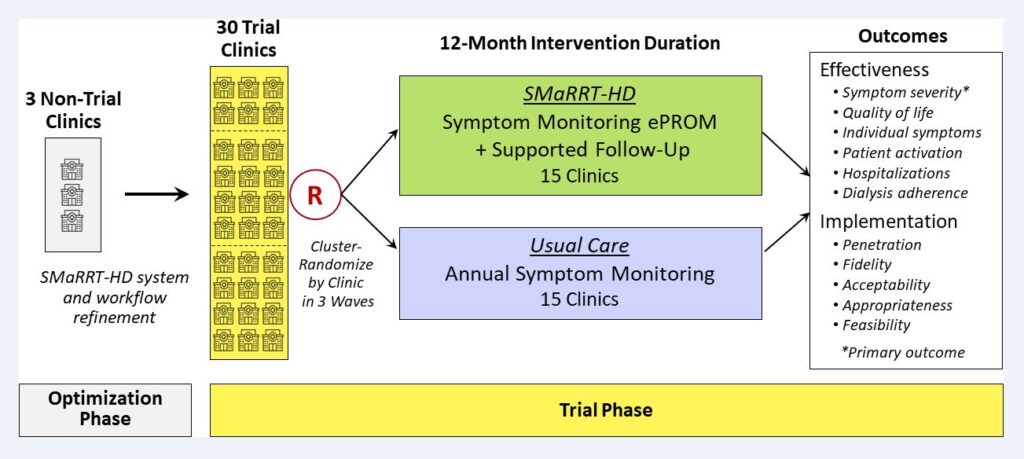
 The SMaRRT-HD Study, also known as “Comparative Effectiveness of Two Approaches to Symptom Monitoring in Hemodialysis” compares two approaches for monitoring and addressing symptoms among adult patients with kidney failure who are treated with hemodialysis. For the first approach patients use an electronic system (called SMaRRT-HD) twice a month to report their symptoms. The system sends alerts to their medical team at the dialysis clinic about the reported symptoms and gives suggestions about how to manage them. The system also provides reports that show patients and their medical team the reported symptoms over time. For the second approach patients complete a quality of life questionnaire that includes questions about symptoms once a year. The questionnaire does not have follow-up support like alerts, symptom management guidance, or reports. In addition, researchers will talk to patients, dialysis clinic personnel, and medical providers about their experiences using the electronic symptom monitoring system to learn about how to best use patient-reported outcome measures in dialysis care.
The SMaRRT-HD Study, also known as “Comparative Effectiveness of Two Approaches to Symptom Monitoring in Hemodialysis” compares two approaches for monitoring and addressing symptoms among adult patients with kidney failure who are treated with hemodialysis. For the first approach patients use an electronic system (called SMaRRT-HD) twice a month to report their symptoms. The system sends alerts to their medical team at the dialysis clinic about the reported symptoms and gives suggestions about how to manage them. The system also provides reports that show patients and their medical team the reported symptoms over time. For the second approach patients complete a quality of life questionnaire that includes questions about symptoms once a year. The questionnaire does not have follow-up support like alerts, symptom management guidance, or reports. In addition, researchers will talk to patients, dialysis clinic personnel, and medical providers about their experiences using the electronic symptom monitoring system to learn about how to best use patient-reported outcome measures in dialysis care.
- Jenny Flythe, Co-Principal Investigator (UNC Chapel Hill)
- Laura Dember, Co-Principal Investigator (University of Pennsylvania)
- Darren DeWalt, Co-Investigator (UNC Chapel Hill)
- In Memoriam, Derek Forfang, Co-Investigator (Patient)
- Laura Hanson, Co-Investigator (UNC Chapel Hill)
- Jesse Hsu, Co-Investigator (University of Pennsylvania)
- Mark Unruh, Co-Investigator (University of New Mexico)
- Virginia Wang, Co-Investigator (Duke University)
- Lorien Dalrymple, Co-Investigator (Fresenius Medical Care)
- Debra Meade, Lead Contact (Fresenius Medical Care)
- Cassandra “Cassie” Picataggio, Research Projects Manager (UNC Chapel Hill)
- Amber Erskine, Research Coordinator (UNC Chapel Hill)
- Emmie Cole, Research Coordinator (UNC Chapel Hill)
- Patients and family members
- Shirley Franks, LPN (Pittsboro, NC)
- Taya Joseph, BA (Raleigh, NC)
- Precious McCowan, MS (Lancaster, TX)
- John Ortiz, DHSc (Riverside, IL)
- Dialysis organization leaders
- Wael Hussein, MD (Satellite Healthcare)
- Robert Kossmann, MD (Fresenius Medical Care)
- Francesca Tentori, MD (DaVita Kidney Care)
- Suzanne Watnick, MD (Northwest Kidney Centers)
- Interdisciplinary care team members
- Shawna Chin (Patient Care Technician, Fresenius Medical Care)
- Frankie Densmore, BSN, RN (Nurse, Fresenius Medical Care)
- Jessica Farrell, MSW (Social Worker, Fresenius Medical Care)
- David Henner, MD (Kidney Doctor, Berkshires Health System)
- Beth Shanaman, RD (Dietitian, Northwest Kidney Centers)
- Policymakers
- Centers for Medicare and Medicaid Services representative
- Learn more about stakeholder panel activities
- Shirley Franks, LPN (Pittsboro, NC)
- Antonio Johnson (Snow Camp, NC)
- Taya Joseph, BA (Raleigh, NC)
- Nathaniel Kirby (Galveston, TX)
- Precious McCowan, MS (Lancaster, TX)
- John Ortiz, DHSc (Riverside, IL)
- Lacye Trevino (Fort Worth, TX)
- Learn more about workgroup activities
- Mehrotra R, Davison SN, Farrington K, Flythe JE, Foo M, Madero M, Morton RL, Tsukamoto Y, Unruh ML, Cheung M, Jadoul M, Winkelmayer WC, Brown EA; Conference Participants. Managing the symptom burden associated with maintenance dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2023 Sep;104(3):441-454. doi: 10.1016/j.kint.2023.05.019. Epub 2023 Jun 7. PMID: 37290600.
- Kidney Disease Improving Global Outcomes (KDIGO) held a Controversies Conference-Symptom-Based Complications in Dialysis-to identify the optimal means for diagnosing and managing symptom-based complications in patients undergoing maintenance dialysis. Participants included patients, physicians, behavioral therapists, nurses, pharmacists, and clinical researchers.
- This article reports on the conference findings. Participants outlined foundational principles and consensus points related to identifying and addressing symptoms experienced by patients undergoing dialysis and described gaps in the knowledge base and priorities for research.
- Flythe JE, Dorough A, Narendra JH, Forfang D, Hartwell L, Abdel-Rahman E. Perspectives on symptom experiences and symptom reporting among individuals on hemodialysis. Nephrol Dial Transplant. 2018 Oct 1;33(10):1842-1852. doi: 10.1093/ndt/gfy069. PMID: 29672712; PMCID: PMC6887921.
- Concept elicitation (patient and clinic personnel interviews about symptoms)
- The goal of this study was to better understand patient experiences with symptoms and symptom reporting. Interviews with patients and dialysis clinic personnel suggested that symptom reporting by patients is highly variable and likely influenced by many personal, treatment and environmental factors.
- Flythe JE, Dorough A, Narendra JH, Wingard RL, Dalrymple LS, DeWalt DA. Development and content validity of a hemodialysis symptom patient-reported outcome measure. Qual Life Res. 2019 Jan;28(1):253-265. doi: 10.1007/s11136-018-2000-7. Epub 2018 Sep 18. PMID: 30229532; PMCID: PMC6339833.
- Cognitive testing (patient interviews about the draft symptom questionnaire)
- The goal of this study was to describe the process and preliminary development of the SMaRRT-HD symptom patient-reported outcome measure (PROM). Interviews with patients provided evidence of the content validity of SMaRRT-HD.
- Jennifer E. Flythe, Matthew J. Tugman, Julia H. Narendra, Adeline Dorough, Johnathan Hilbert, Magdalene M. Assimon, Darren A. DeWalt. Feasibility of Tablet-Based Patient-Reported Symptom Data Collection Among Hemodialysis Patients, Kidney International Reports, Volume 5, Issue 7, 2020, Pages 1026-1039, ISSN 2468-0249.
- Pilot test (real-world implementation of SMaRRT-HD)
- The goal of this study was to evaluate the preliminary efficacy and feasibility of using the SMaRRT-HD symptom monitoring system in routine hemodialysis care. The pilot test demonstrated that using the SMaRRT-HD system during routine hemodialysis care is feasible and may improve communication about symptoms between patients and their dialysis care teams.
- Flythe JE, Narendra JH, Hilliard T, Frazier K, Ikeler K, Amolegbe A, Mitchell D, Dorough A, Lee SD, Ordish A, Wilkie C, Dember LM; Cultivating a Research-Ready Dialysis Community. J Am Soc Nephrol. 2019 Mar;30(3):375-380. doi: 10.1681/ASN.2018101059. Epub 2019 Feb 8. PMID: 30737271; PMCID: PMC6405153.
- Dialysis community research capacity (principles underlying our approach to stakeholder engagement)
- This article reports consensus findings from a stakeholder workshop about building research capacity in the dialysis setting. Key components of a research-ready dialysis clinic environment include sufficient knowledge, meaningful stakeholder engagement, transparent and consistent communication, and research processes that minimize the burden of research in the setting of clinical care.
- Basch E, Rocque G, Mody G, Mullangi S, Patt D. Tenets for Implementing Electronic Patient-Reported Outcomes for Remote Symptom Monitoring During Cancer Treatment. JCO Clin Cancer Inform. 2023 Feb;7:e2200187. doi: 10.1200/CCI.22.00187. PMID: 36857630.
- Best practices for implementing symptom monitoring systems in cancer
- In July 2023, the use of electronic patient-reported outcomes (ePROs) will become required in oncology practice as part of a new payment model. This article summarizes best practices for implementing ePRO symptom monitoring systems in cancer. We aim to incorporate relevant principles into our implementation of the SMaRRT-HD system in dialysis care.
Patient-Centered Outcomes Research Institute (PCORI) Research Award (IHS-2021C2-23534)
Contact
Questions? Comments? We want to hear from you!
Email smarrt-hd@unc.edu or call 1-888-804-9511